New Product Launch | NEX-t Panel: A More Precise and Cost-effective tNGS Pathogen Detection Solution
01 Background
Targeted Next-Generation Sequencing (tNGS) is a widely used technology for the detection of pathogen microorganisms. There are primarily two approaches: one involves enriching target sequences using multiplex amplification to ganerate sequencing libraries, while the other adds upon metagenomic Next-Generation sequencing (mNGS) by incorporating a hybridization step with probe, targeting the capture of mNGS libraries to enrich pathogen sequences.
The former approach allows for multiplex amplification of thousands of targets, making it relatively simple and fast. However, its stability is comparatively lower, resulting in a certain rate of amplification failure. The latter approach offers greater scalability, with current solutions on the market utilizing probes sets in millions to provide detection breadth comparable to mNGS. This approach employs forward selection to addresses a major issue of mNGS: the removal of host sequences.
02 Overview
NEX-t Panel v1.0 is a tNGS panel based on probe hybridization capture. It targets a series of characteristic sequences selected from the genomes of hundreds of pathogens including viruses, bacteria, fungi and parasites. It covers a rich range of content including 16S/ITS, housekeeping genes, drug-resistant related genes, and more. By fully leveraging the fault-tolerant advantages of probe hybridization, the panel size has been streamlined to approximately 8,000 probes, maintaining enrichment information while reducing the complexity of implementation.
NEX-t Panel v1.0 is recommended for use in conjunction with NadPrep ES Hybrid Capture Reagents, which significantly shorten the hybridization time while simplifying the experimental steps.
2.1 Solution Design
NEX-t Panel v1.0 primarily consists of the following three components:
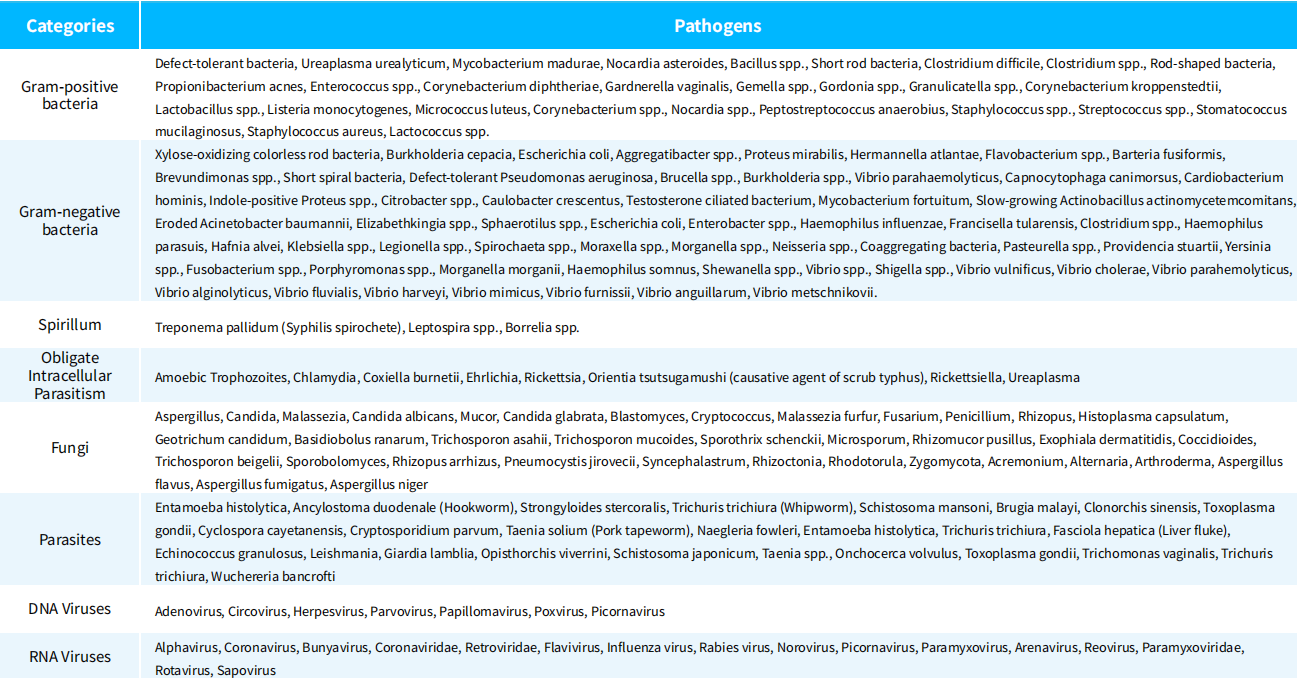
(1) Probes designed for characteristic sequences of hundreds of species, including viruses, bacteria, fungi, and parasites, as listed in Table 1.
l Comparison of multiple reference sequences within species or genera, selecting regions within probe tolerance range and containing certain a degree of diversity.
l Incorporation of sequences involved in Multi-Locus Sequence Typing (MLST).
l Integration of relevant literature.
(2) A small set of universal probes designed for 16S/ITS.
l Reference from multiple databases (SILVA, Greengenes, RDP, Unite).
(3) Probes designed for drug resistance, virulence, and related genes.
Table 1. Major Pathogenic Species Covered by NEX-t Panel v1.0
2.2 Recommended Analysis Workflow
The analysis of captured data from NEX-t Panel v1.0 can initiated from the sequences captured by the probes in the first part. This analysis involves identifying the pathogenic species listed in Table 1. Furthermore, from the sequences captured by the 16S/ITS probes in the second part, the search can be extended to identify any other microorganisms not included in Table 1. For specific pathogens, additional information regarding drug resistance and virulence can be extracted from the sequences captured by the probes in the third part (refer to Figure 1).
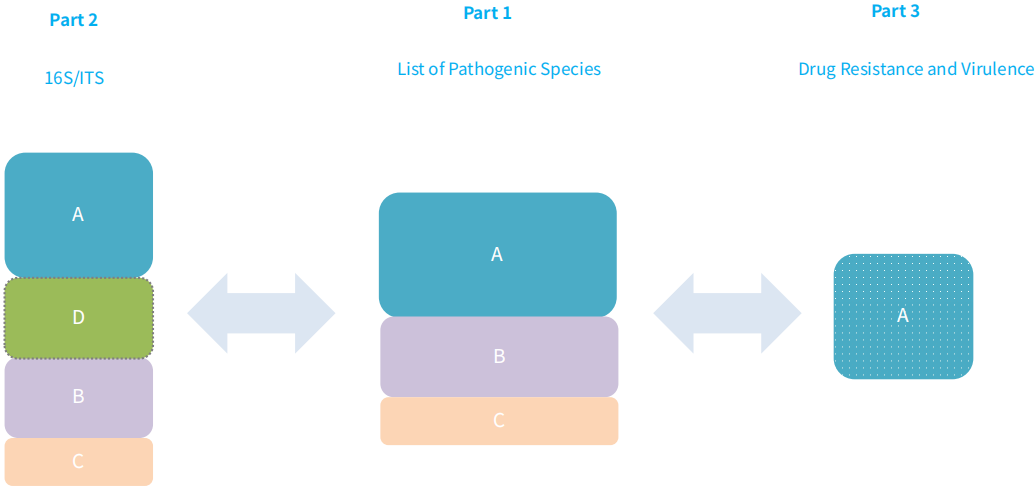
Figure 1. NEX-t Panel v1.0 capture data analysis
03 Feature and Performance
3.1 Importance of Design over Quantity in tNGS Probes
In general, achieving complete probe coverage of pathogen microbiome genomes is impractical. On one hand, designing probes for a single bacterial species can result in hundreds of thousands of probes when considering sequence polymorphism beyond reference genomes. On the other hand, to prevent off-target effects when there is similarity between pathogenic and host sequences, certain regions must be excluded. Therefore, NEX-t Panel v1.0 emphasizes designing the fewest probes to enable the broadest range of pathogen analysis, resulting in a significantly streamlined panel size compared to tNGS schemes with millions of probes in hybrid capture.
The number of probes can be roughly equivalent to the number of amplicons in multiplex PCR. However, due to the higher tolerance of probes and the ability to capture sequences flanking the probe, a certain number of probes can provide more diverse information compared to a similar number of multiplex amplicons.
Compared to multiplex PCR, expanding a hybrid capture panel is simpler as it only requires adding probes. Therefore, NEX-t Panel v1.0 can be conveniently customized and upgraded through combination.
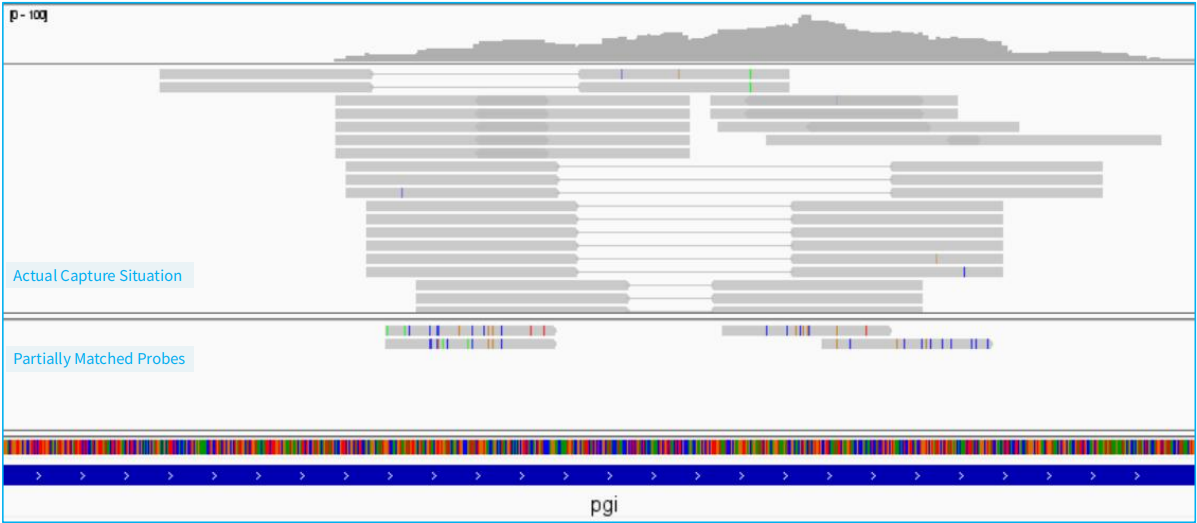
Figure 2. Example of probe tolerance in NEX-t Panel v1.0 (Non-design regions)
3.2 Close Relationship Between On-target Rate and Pathogen Copy Number
The on-target rate of pathogens are significantly influenced by the amount of pathogens within the samples. As shown in Figure 3, under the same host background, as the microbial reference material is gradually diluted, the amount of data decreases proportionally, along with a decrease in on-target rate.
Although multiple capture rounds can substantially improve on-target rate, this strategy comes with the risk of information loss (dropout). Especially when the amount of pathogen is low, the on-target rate is low and is more l sensitive to dropout. Therefore, careful consideration of the pros and cons is needed when contemplating multiple capture rounds. Additionally, from another perspective, when the pathogen genome copy numbers in the sample are in the single digits, increasing on-target rate merely involves repetitive sequencing of these few copies, yielding limited additional useful information.
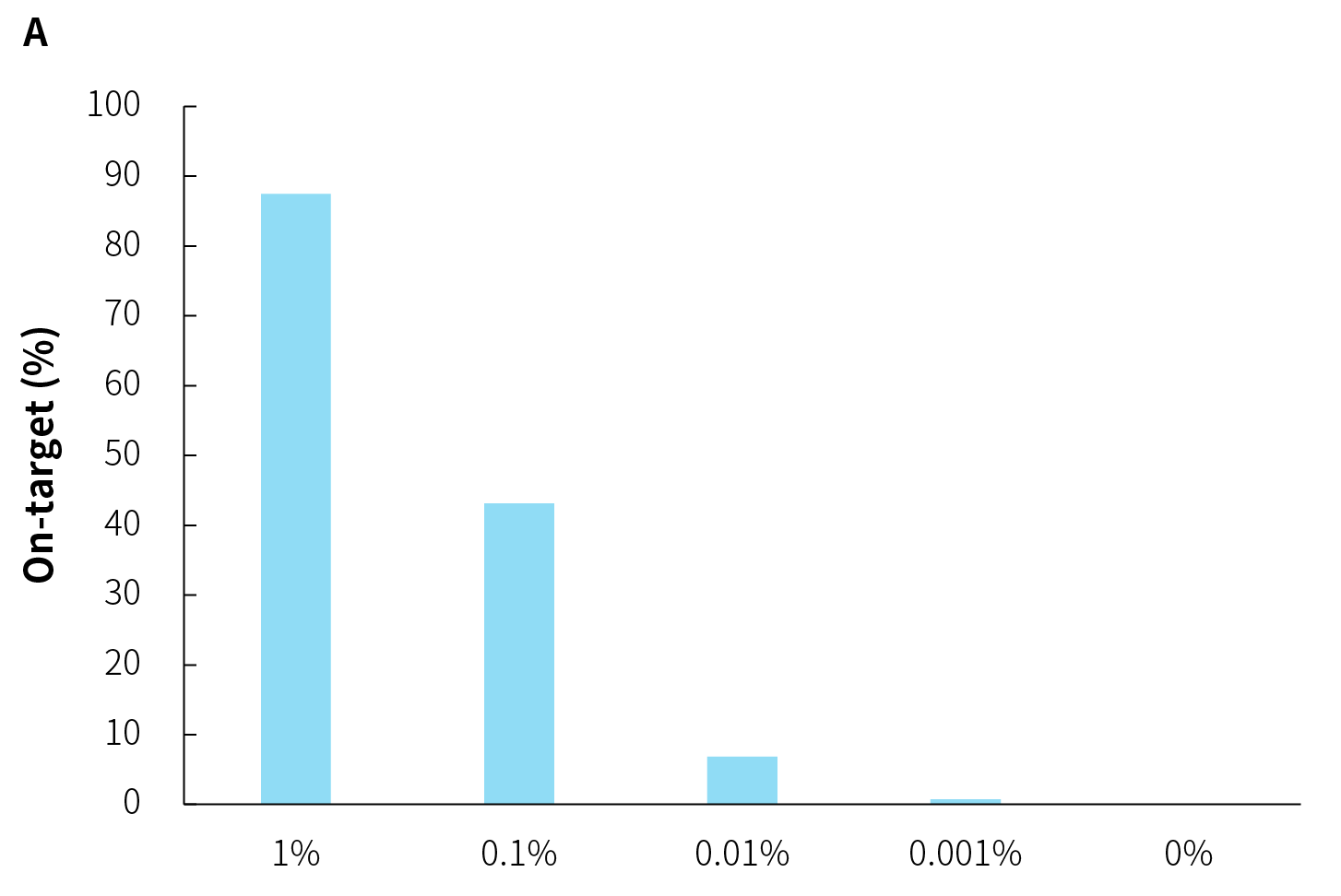
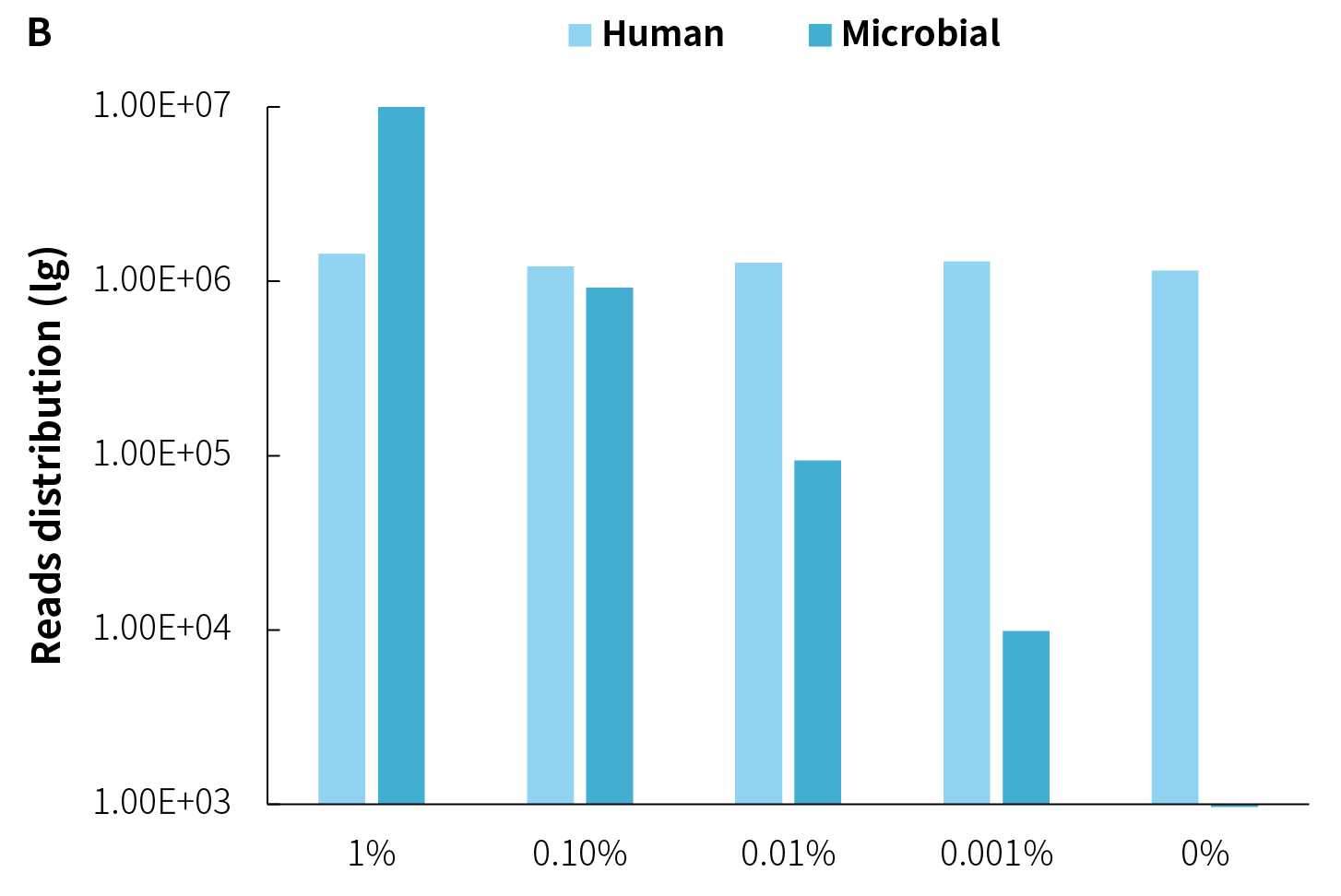
Figure 3. On-target rate and reads distribution of NEX-t Panel v1.0 across different samples. A. On-target rate; B. Captured reads distribution.
Note:
① Simulated microbial community samples of 0.001% - 1% MSA-1003 were created by diluting a mixture of 20 strains of genomic material (ATCC, MSA-1003) using the human genomic DNA standard (Promega, G1471) at various ratios.
② Library preparation was performed using 50 ng input with the NadPrep EZ DNA Library Preparation Kit v2, followed by hybrid capture (2-hour hybridization) using NEX-t Panel v1.0 with NadPrep ES Hybrid Capture Reagents.
③ Sequencing platform: Illumina Novaseq6000, PE150. The BWA was used for alignment of raw reads to the reference genome composed of the hg19 human genome and 20 microbial genome reference sequences, and reads distribution was analyzed.
3.3 Detection Sensitivity
As shown in Figure 4, the detection limit for samples with varying microbial content is approximately 3 copies. In practical applications, sensitivity is mainly determined by the amount of input for library preparation. It's important to note that the library preparation process is constrained by conversion efficiency, meaning not all copies can be successfully converted. Similarly, when the copy number is in the single digits, the success rate of multiplex amplicons' amplification decreases.
Furthermore, having less than 1 copy does not imply that it cannot be detected. This is due to the fact that when NEX-t Panel v1.0 features multiple probe capture regions for a single species, their copy numbers can accumulate, enhancing the detection sensitivity.
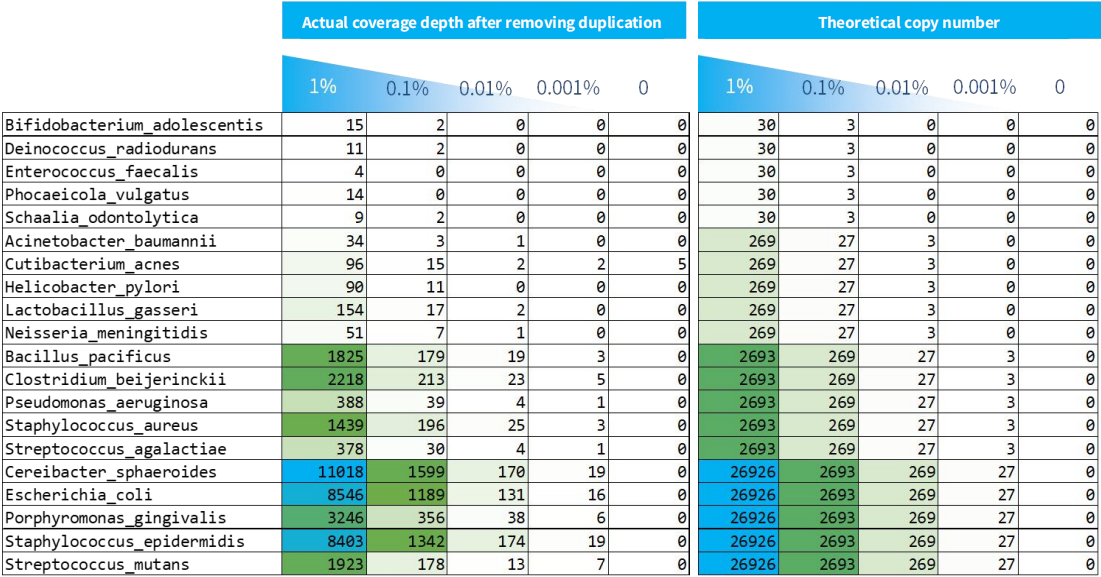
Figure 4. Captured coverage depth after duplication removal of NEX-t Panel v1.0 for samples with different microbial content.
3.4 Multiple-plex Hybridization
Due to significant variations in pathogen content among different samples, if the aim is to achieve similar data amounts for each sample, it’s not feasible to perform multiple-plex hybridization; separate hybridization must be carried out instead. However, as shown in Figure 5, when there are orders of magnitude differences in microbial content among samples, although there are substantial variations in data amounts, low-abundance samples require relatively less data on their own. Moreover, different samples, using the same experimental parameters, exhibit similar PCR duplication rates. If they are subjected to separate hybrid capture and provided with the same sequencing data, low-abundance samples would need an increased number of PCR cycles. The identical information content would only be repeated to form an augmented sequencing data, as post-panel capture sequencing becomes easier to saturate. Based on these considerations, for samples with similar origins, performing the multiple-plex hybridization process is recommended.
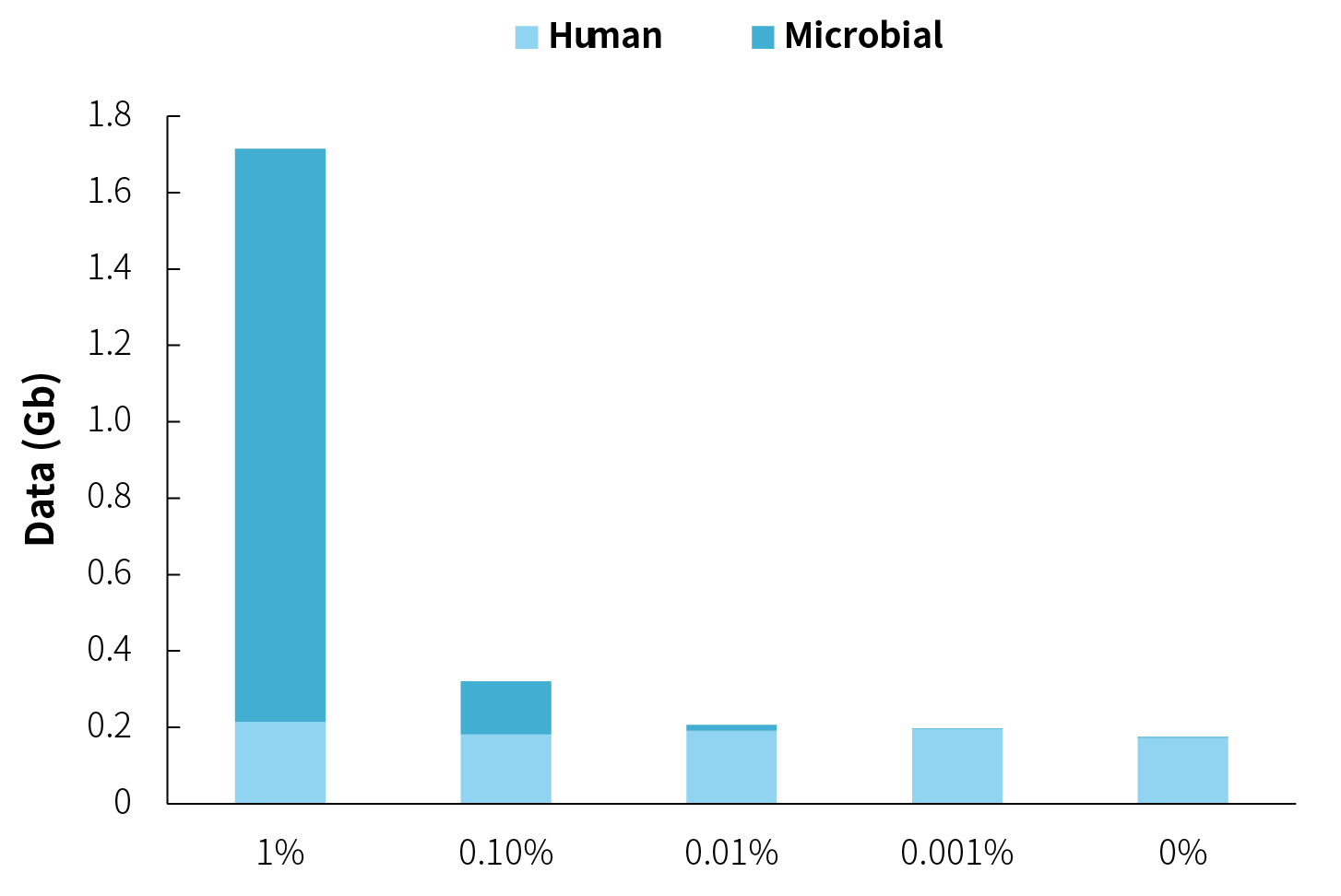
Figure 5. Capture data amounts for NEX-t Panel v1.0 using multiple-plex hybridization for microbial samples with different orders of magnitude
3.5 Simultaneous Hybridization Capture of RNA and DNA Viruses
Different library preparation approaches can be employed for RNA and DNA viruses. One approach involves the concurrent library preparation of RNA and DNA viruses, followed by subsequent hybridization capture steps. Another approach entails preparing RNA libraries and DNA libraries separately, and then mixing them for hybridization capture. Generally, for RNA libraries, there is no need to perform the step of removing of host ribosomal RNA.
As shown in Figure 6, the example demonstrates the capture scenario when RNA libraries containing influenza virus (H10N3) and DNA libraries are hybridized together. A total of 15 libraries are involved, including 11 DNA libraries and 4 RNA libraries. The example library data amount was 100 Mb (totaling 9.3 Gb for all 15 libraries), with host sequences accounting for 58.3%.
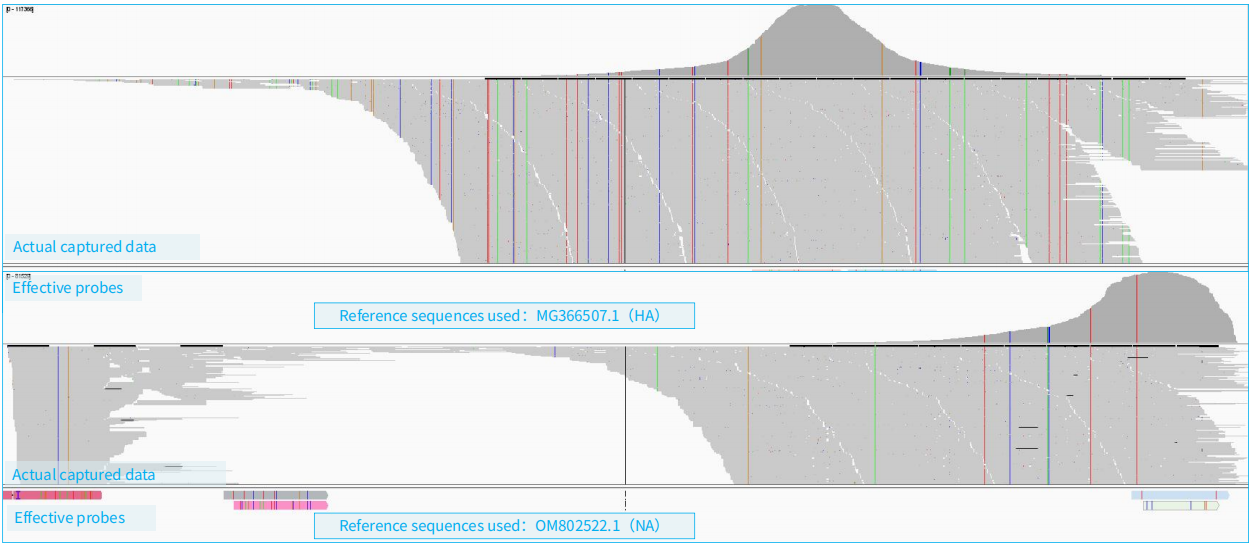
Figure 6. Simultaneous Capture of RNA and DNA Viruses using NEX-t Panel v1.0
04 Conclusion
Both mNGS and tNGS are effective tools for infection detection, but they serve different scenarios. Clinically, appropriate technologies can be chosen based on patient needs to assist in precise diagnosis and treatment. The introduction of NEX-t Panel v1.0 offers a new choice between large-scale hybrid capture tNGS and multiplex amplicon tNGS. It is expected to provide a more precise and cost-effective solution for tNGS detection. It holds the potential to play a significant role in clinical practice.
Solutions
- Methyl Library Preparation Total Solution
- Sequencing single library on different platform--Universal Stubby Adapter (UDI)
- HRD score Analysis
- Unique Dual Index for MGI platforms
- RNA-Cap Sequencing of Human Respiratory Viruses Including SARS-CoV-2
- Total Solution for RNA-Cap Sequencing
- Total Solution for MGI Platforms
- Whole Exome Sequencing
- Low-frequency Mutation Analysis
Events
-
Exhibition Preview | Nanodigmbio invites you to join us at Boston 2025 Annual Meeting of the American Society of Human Genetics (ASHG)

-
Exhibition Preview | Nanodigmbio Invites You to Join Us at WHX & WHX Labs Kuala Lumpur 2025, Malaysia International Trade and Exhibition Centre in Kuala Lumpur

-
Exhibition Preview | Nanodigmbio Invites You to Join Us at Hospitalar 2025, Brazil International Medical Device Exhibition in São Paulo

-
Exhibition Preview | Nanodigmbio invites you to join us at Denver 2024 Annual Meeting of the American Society of Human Genetics (ASHG)

-
Exhibition Preview | Nanodigmbio invites you to join us at Sapporo 2024 Annual Meeting of the Japan Society of Human Genetics (JSHG)

-
Exhibition Preview | Nanodigmbio invites you to join us at Association for Diagnostics & Laboratory Medicine (ADLM)



