End-to-End Solution for PGT-A by LP-WGS
Background
Preimplantation genetic testing
for Aneuploidy (PGT-A) is a genetic
screening procedure performed on embryonic cells obtained by in vitro
fertilization (IVF) prior to uterine transfer, with the aim of identifying
chromosomal aneuploidies and selecting embryos suitable for transfer. Recent
studies indicate that PGT-A can significantly improve implantation rate,
clinical pregnancy rate, ongoing pregnancy rate and live birth rate in women of
advanced maternal age, while reducing the risks of miscarriage and congenital
anomalies and decreasing the vertical transmission risk of hereditary disorders[1-3].
PGT-A technologies have evolved from early
fluorescence in situ hybridization (FISH) to microarray platforms (aCGH/SNP
array) and, more recently, to widely adopted next-generation sequencing (NGS) methods.
With advances in blastocyst culture and molecular diagnostics, NGS owing to its
higher resolution and accuracy, has gradually become the preferred choice for
PGT-A in reproductive medicine[4-5]. In clinical practice, PGT-A
commonly employs low-pass whole-genome sequencing (LP-WGS) to generate read-depth
information across the whole genome that can be analyzed to detect
whole-chromosome aneuploidies, segmental aneuploidies, and, to a certain
extent, mosaicism.
Responding to these technological advances and clinical demands, Nanodigmbio developed the End-to-End Solution for PGT-A by LP-WGS. By integrating optimized whole-genome amplification (WGA), NGS library preparation, and bioinformatics analysis pipelines, this solution provides an integrated workflow from single-cell or ultra-low input gDNA samples through to automated reporting, enabling laboratories to increase throughput and turnaround while maintaining sensitivity and accuracy, thereby delivering reliable technical support for reproductive decision-making.
Solution
End-to-End
Solution for PGT-A by LP-WGS (hereafter referred to as Comprehensive PGT-A Solution) provides a
streamlined, scalable workflow for NGS-based PGT-A using LP-WGS. The solution
integrates the NadPrep Single Cell WGA Kit, NadPrep DNA Library
Preparation Kit series with XCapViz Bioinformatics Visualization System and prebuilt analysis pipeline (SenS PGT-A) to enable automated
reporting and interpretation.
Comprehensive PGT-A Solution has been optimized for the reliable detection of CNVs, including whole-chromosome, mosaic and segmental aneuploidies, from DNA derived from single cells or ultra-low amounts of purified gDNA. The complete workflow begins with cell lysis and WGA, followed by NGS library preparation and incorporation of unique dual indexes (UDIs). Final libraries can be pooled for multiplexed sequencing of up to 768 samples per run on mainstream sequencing platforms. Due to the prebuilt pipeline in XCapViz, Comprehensive PGT-A Solution delivers accurate calls of aneuploidies, reliable detection of low-level mosaicism (~30%) in defined mixtures, and the capability to call chromosomal aneuploidies at 5 Mb-resolution with as few as 5 M read pairs. By delivering high-quality, reproducible data, Comprehensive PGT-A Solution is expected to become a powerful technological tool to gain deeper insights into chromosomal variation in PGT-A applications.
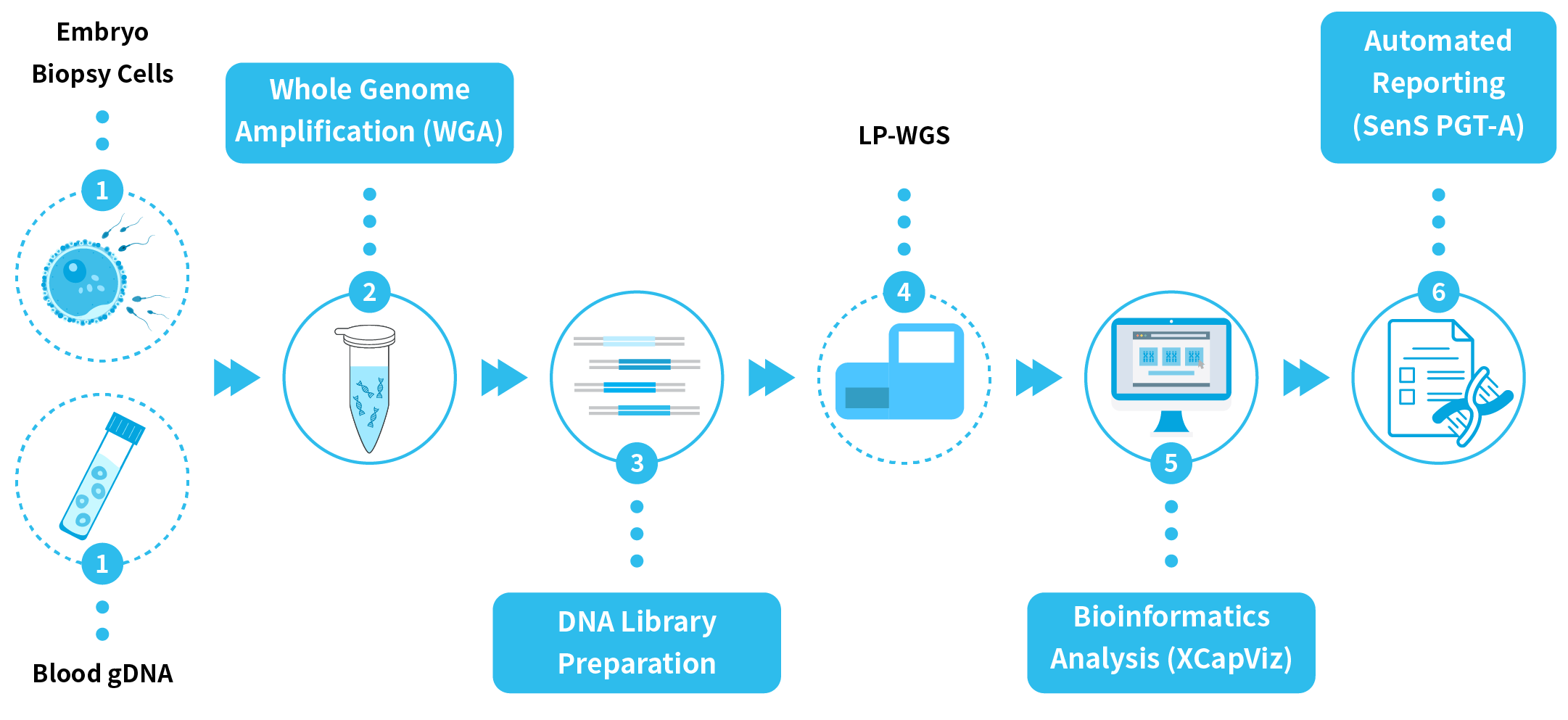
| Single Cell WGA | DNA Library Preparation | Bioinformatics Analysis |
| NadPrep Single Cell WGA Kit |
NadPrep DNA Library Preparation Kit v2 or |
• XCapViz Bioinformation Analysis Visual System • Prebuilt Pipelines (SenS PGT-A) |
For research use only. Not for use in diagnostic procedures.



Note: Samples were cell line-derived gDNA reference standards characterized by pronounced trisomy 13/18/21 (LDT Bioscience, C2337T/C2338T/C2339T).
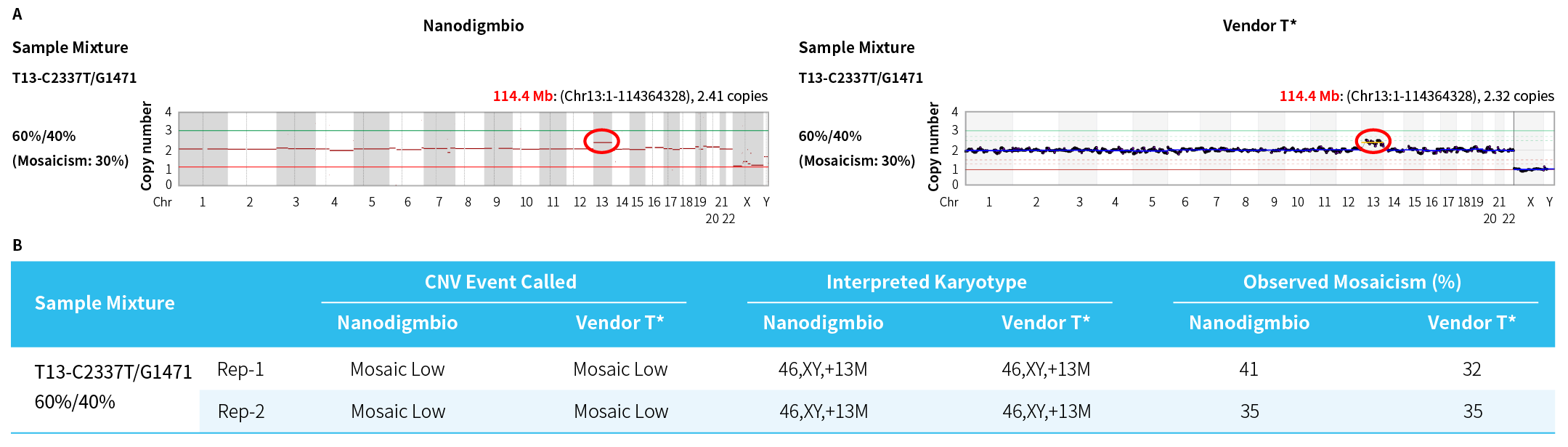
Figure 2. Evaluation of the minimum detectable level of mosaicisms in mosaic samples demonstrates concordance between results from Comprehensive PGT-A Solution and Vendor T*. A. Scatter plot of whole-chromosome copy number; B. Calling and interpretation of chromosomal mosaicisms. All the CNVs analysis and interpretation was performed with SenS PGT-A in 100-kb bin sizes.
Note: Artificial mosaic samples were generated by mixing two gDNA standards [C2337T and G1471 (Promega)] at the indicated ratio, containing mosaic aneuploidies at 30% across chromosome 13.
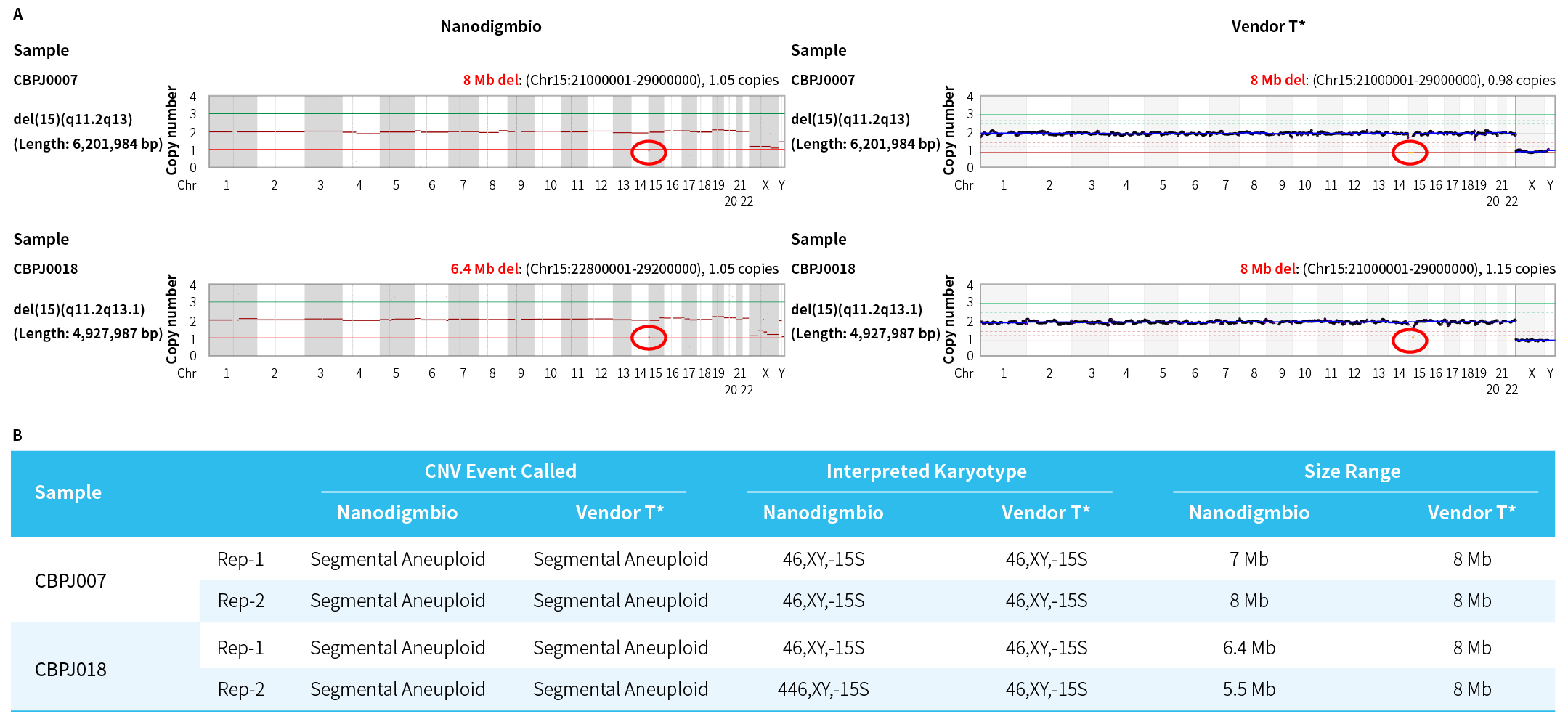
Figure 3. Comprehensive PGT-A Solution demonstrates higher resolution in detecting segmental aneuploidies compared to Vendor T* in standards with known chromosomal abnormalities. A. Scatter plot of whole-chromosome copy number; B. Calling and interpretation of chromosomal segmental aneuploidies.
Note: Samples were Prader-Willi syndrome (46,XY,del(15)(q11.2q13)) Reference Standard (COBIOER, CBPJ0007) and Prader-Willi syndrome (46,XY,del(15)(q11.2q13.1)) Reference Standard (COBIOER, CBPJ0018).
Complete Solution for PGT-A by
LP-WGS
• Rapid: sample-to-insight
TAT commonly in less than 12 hr
• High-throughput: supports
multiplexing of up to 768 samples in a single run
• Flexible: broadly
compatible with mainstream sequencing platforms
• Automated: streamlined,
user-friendly analysis pipeline with reporting and interpretation
Accurately Detection and
Calling of Aneuploidies
• Reliably calls whole-chromosome and segmental
aneuploidies down to a size of 10 Mb, as well as low (30%) mosaicisms for
defined mixtures of samples with known aneuploidies.
• Capable of calling chromosomal deletion or duplication
at 5 Mb-resolution with as few as 5 M read pairs.
Reference
[1] Kim J G, Murugappan G, Lathi R B, et al.
Preimplantation genetic testing for aneuploidy (PGT-A) reduces miscarriage and
improves live birth rates in recurrent pregnancy loss patients[J]. Fertility
and Sterility, 2019, 112(3): e401.
[2] Munné S, Kaplan B,
Frattarelli J L, et al. Preimplantation genetic testing for aneuploidy versus
morphology as selection criteria for single frozen-thawed embryo transfer in
good-prognosis patients: a multicenter randomized clinical trial[J]. Fertility
and sterility, 2019, 112(6): 1071-1079. e7.
[3] Chang J, Boulet S L, Jeng G, et al. Outcomes of
in vitro fertilization with preimplantation genetic diagnosis: an analysis of
the United States Assisted Reproductive Technology Surveillance Data, 2011–2012[J]. Fertility and sterility, 2016, 105(2): 394-400.
[4] Sciorio R, Tramontano L, Catt J.
Preimplantation genetic diagnosis (PGD) and genetic testing for aneuploidy
(PGT-A): status and future challenges[J]. Gynecological Endocrinology, 2020,
36(1): 6-11.
[5] Chen H F, Chen M, Ho H N. An overview of the current and emerging platforms for preimplantation genetic testing for aneuploidies (PGT-A) in in vitro fertilization programs[J]. Taiwanese Journal of Obstetrics and Gynecology, 2020, 59(4): 489-495.
Solutions
- Methyl Library Preparation Total Solution
- Sequencing single library on different platform--Universal Stubby Adapter (UDI)
- HRD score Analysis
- Unique Dual Index for MGI platforms
- RNA-Cap Sequencing of Human Respiratory Viruses Including SARS-CoV-2
- Total Solution for RNA-Cap Sequencing
- Total Solution for MGI Platforms
- Whole Exome Sequencing
- Low-frequency Mutation Analysis
Events
-
Exhibition Preview | Nanodigmbio invites you to join us at Boston 2025 Annual Meeting of the American Society of Human Genetics (ASHG)

-
Exhibition Preview | Nanodigmbio Invites You to Join Us at WHX & WHX Labs Kuala Lumpur 2025, Malaysia International Trade and Exhibition Centre in Kuala Lumpur

-
Exhibition Preview | Nanodigmbio Invites You to Join Us at Hospitalar 2025, Brazil International Medical Device Exhibition in São Paulo

-
Exhibition Preview | Nanodigmbio invites you to join us at Denver 2024 Annual Meeting of the American Society of Human Genetics (ASHG)

-
Exhibition Preview | Nanodigmbio invites you to join us at Sapporo 2024 Annual Meeting of the Japan Society of Human Genetics (JSHG)

-
Exhibition Preview | Nanodigmbio invites you to join us at Association for Diagnostics & Laboratory Medicine (ADLM)



